COVID-19 N-Pak Specimen Collection Kits for Upper Respiratory Tract Specimens
March 17, 2020

Due to the recent 2019 Novel Coronavirus (COVID-19) outbreak, we have been getting many inquiries about what is the best collection method for COVID-19 sample collection prior to testing. Below we have a short guide on which N-Pak products meet the Centers for Disease Control and Prevention (CDC) Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for Coronavirus investigations.
Which Method of Specimen Should be Used to Collect Samples to Test for Coronavirus (COVID-19)?
As outlined in the CDC guidelines for the collection and handling of clinical specimens suspected of Coronavirus Disease 2019 (COVID-19), for upper respiratory tract samples, health workers should be aware of the following:
- Collecting diagnostic respiratory specimens (e.g., nasopharyngeal swab) is likely to induce cough or sneezing.
- Non-aerosol-generating procedures should be performed before aerosol-generating procedures
N-Pak is less stimulating than a nasopharyngeal swab and is a non-aerosol-generating collection method. Although the ideal type of specimen for Covid-19 virus continues to be evaluated, the pharynx and nasopharynx appear to be the focus for screening tests at this time. Historically nasopharyngeal aspiration provides the highest sensitivities compared to other collection methods such as NP swab or wash for viral pathogens.
How Should Collection Kits be Used for Coronavirus (COVID-19) Testing?
In the CDC Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons Under Investigation (PUIs) for Coronavirus Disease 2019 (COVID-19), the CDC recommends taking a nasopharyngeal aspirate for COVID-19 for upper respiratory tract samples. The CDC guidelines also direct healthcare workers to have the NP aspirate of 2-3 mL in a sterile, leak-proof, screw-cap sterile dry container therefor after the NP aspirate is obtained using N-Pak, the syringe can simply be capped and labeled. Store specimens at 2-8°C for up to 72 hours after collection. If a delay in testing or shipping is expected, store specimens at -70°C or below[1].
COVID-19 Sample Collection Kits and Components that Meet the CDC’s Guidelines
N-Pak kit and single catheters are compatible with the CDC-recommendations.
Sterile-packaged Syringe Aspiration Kit (SAK-01)
Sterile packaged Single Catheter (CATH-01)
Also, Healthcare Providers can quickly assemble in-house sample collection kits and place the items in a Ziploc/Minigrip bag for distribution using the N-Pak isolated single catheter, (CATH-01) a sterile control syringe, and injectable sterile saline and drawing needle.
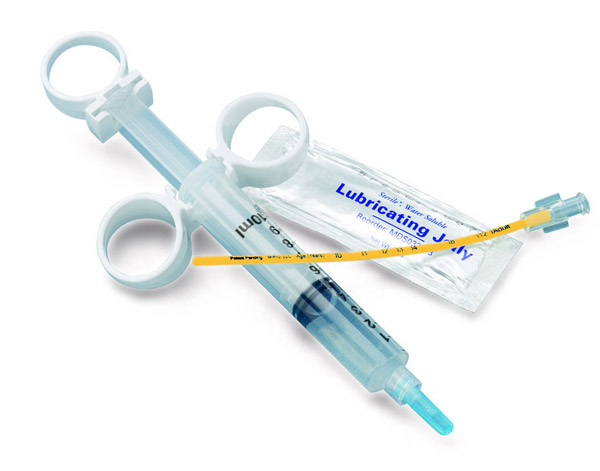

Syringe Aspiration Kit (SAK-01)
Single Catheter (CATH-01)
References
- Centers for Disease Control and Prevention. (2020, February 14). Coronavirus Disease 2019 (COVID-19): Guidelines for Clinical Specimens. Retrieved February 27, 2020, from https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html

